Alkyl Polyglucoside (APG) stands out as an excellent non-ionic surfactant, derived from glucose and natural fatty alcohols. Formed by the reaction between aldehyde hydroxyl groups and alcohol hydroxyl groups, APG boasts advantages such as good surface activity, excellent foaming properties, high compatibility, non-toxicity, and complete biodegradability. These qualities have led to widespread applications in industries like textiles and daily chemicals. This article delves into the molecular structure of different APG variants with varying alkyl chain lengths (APG08(R=C8), APG10(R=C10), APG0810(R=C8-10), APG1214(R=C12-14)), examining how external factors influence their foam performance.
Hydrophobic Chain Impact on APG Foam Performance
Setting the temperature at 25℃, various concentrations of APG surfactants were chosen for foaming experiments, measuring foam height and the time for 50% foam drainage, as shown in Figure 1 and Figure 2.
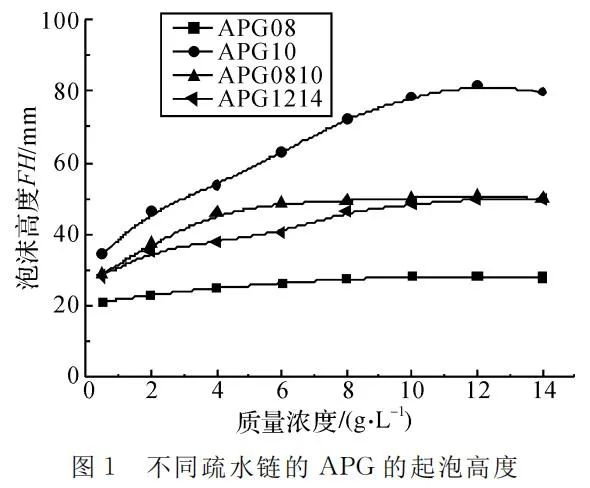
Comparing the four curves in Figure 1, it becomes evident that as the hydrophobicity of APG increases, foaming ability initially rises and then declines, with the best foaming observed at a hydrophobic chain carbon number of 10. The foaming ability of surfactants is related to liquid surface tension, where lower surface tension corresponds to better foaming. However, above the critical micelle concentration, the liquid surface tension of APG surfactants is essentially the same. Foaming ability is also influenced by the Hydrophilic-Lipophilic Balance (HLB) value of surfactants. APG08, with a short hydrophobic chain, has a high HLB value and poorer foam performance, while APG1214, with a longer hydrophobic chain and lower HLB value, also exhibits weaker foaming. APG10, with a moderately balanced HLB value, shows better foam performance.
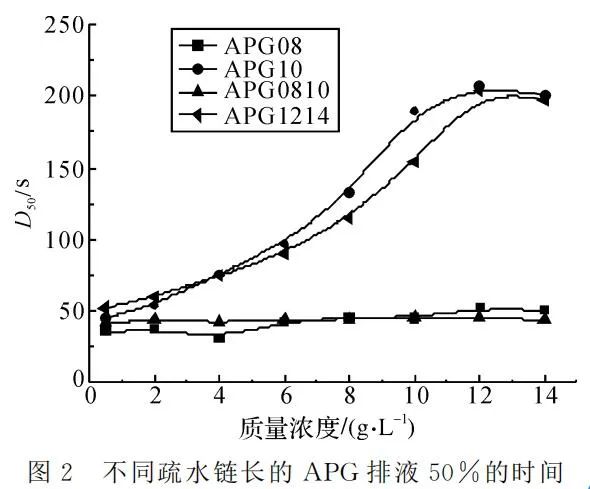
By comparing the four curves in Figure 2, the impact of the hydrophobic chain on the stable foaming of APG becomes evident. Longer hydrophobic chains contribute to better foam stability. A longer hydrophobic chain allows the formation of a tight adsorption layer on the liquid film surface of bubbles, increasing the surface viscosity of the foam. This slows down the diffusion rate of gas through the liquid film. Additionally, a longer hydrophobic chain increases the liquid phase viscosity of the APG foaming solution, slowing down the liquid drainage rate in the foam. This, in turn, is beneficial for foam stability. However, if the carbon chain is too long, the formed foam surface film's rigidity becomes too strong, hindering foam stability.
Temperature Impact on APG Foam Performance
Controlling the APG mass concentration at 4g/L, foaming height and the time for 50% foam drainage were measured at different temperatures, as shown in Figure 3 and Figure 4.
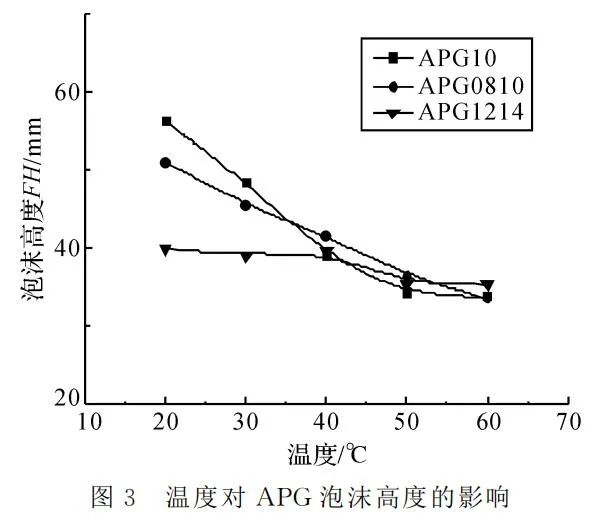
Figure 3 illustrates that as the temperature rises, the foaming ability of APG surfactants decreases, but the degree of decrease varies with different hydrophobic chain lengths. APG0810 and APG10 show a significant decrease, while APG1214 exhibits a smaller decline. In general, an increase in temperature results in a decrease in the foaming ability of APG. However, with a longer hydrophobic chain, the relative increase in resistance to molecular movement of surfactants is greater, leading to a relatively slower decline in foaming ability.
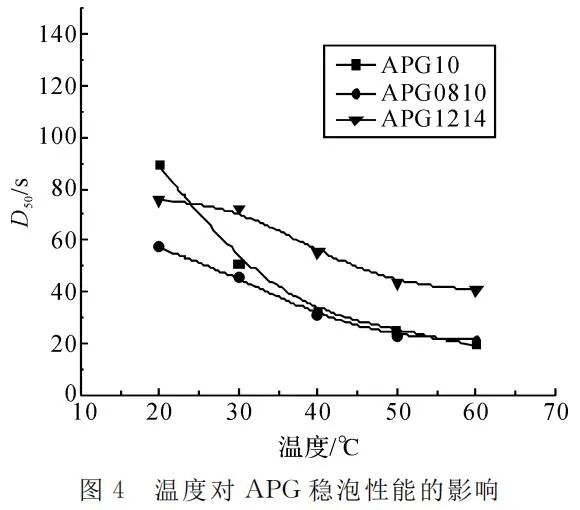
Figure 4 demonstrates that an increase in temperature lowers the time for 50% foam drainage of APG surfactants, indicating a decrease in foam stability. Temperature significantly influences the properties of the foam liquid film. As temperature rises, the adsorption amount of surfactants on the gas-liquid interface decreases, leading to a reduction in the surface viscosity and surface elasticity of the foam. Consequently, foam stability decreases.
Effect of Sodium Sulfate on APG Foam Performance
Controlling the temperature at 25℃ and APG mass concentration at 4g/L, the foam height and the time for 50% foam drainage were measured in sodium sulfate solutions of different concentrations, as shown in Figure 5 and Figure 6.
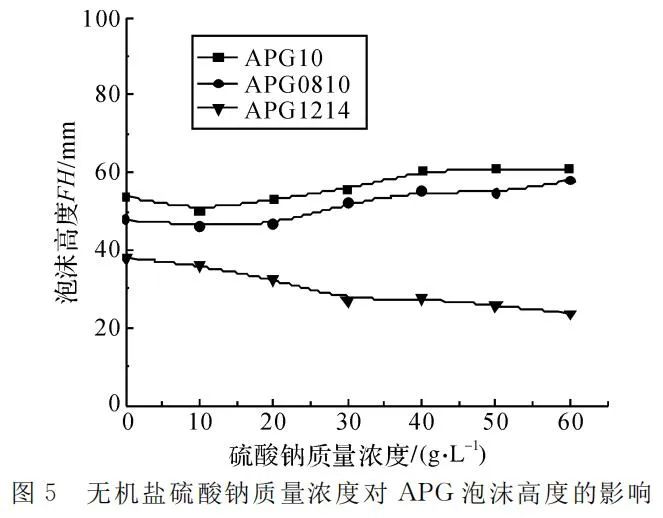
Figure 5 reveals that the addition of sodium sulfate affects the foaming ability of APG differently. With an increase in sodium sulfate concentration, the foaming ability of APG1214 gradually decreases, while that of APG10 and APG0810 increases. Inorganic salts increase the surface tension of the liquid, which is disadvantageous for the foaming of the foaming solution. However, APG surfactants exhibit a certain negative charge in the solution, and the addition of sodium sulfate introduces counterions (Na+), promoting the ordered arrangement of APG molecules. This, in turn, reduces the surface tension of the liquid and favors foaming.
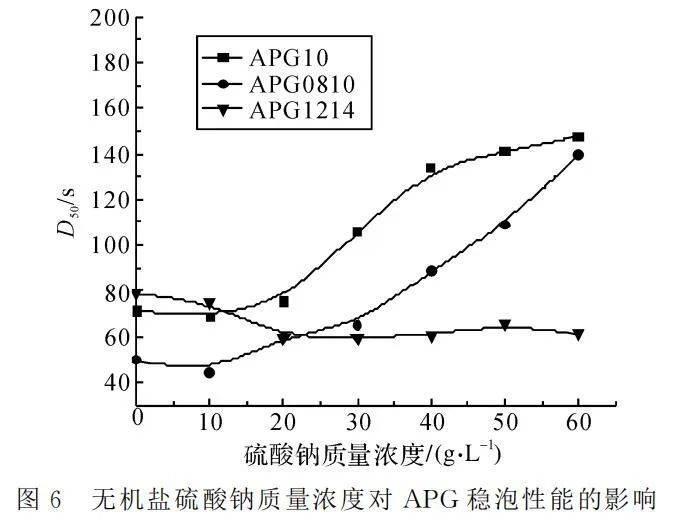
Figure 6 demonstrates that the influence of inorganic salt concentration on the stable foaming of different APG variants varies. The stable foaming of APG0810 and APG10 significantly increases, while that of APG1214 decreases. The addition of sodium sulfate enhances foam stability. However, when the hydrophobic chain is longer, the negative charge of APG surfactants is weaker, and the counterion effect is weaker. With a higher concentration of inorganic salt, the surface tension of the liquid increases, resulting in higher energy of the foam system and lower stability.
Impact of System pH on APG Foam Performance
Setting the temperature at 25℃ and APG mass concentration at 4g/L, foam height and the time for 50% foam drainage were measured under different pH conditions, as shown in Figure 7 and Figure 8.
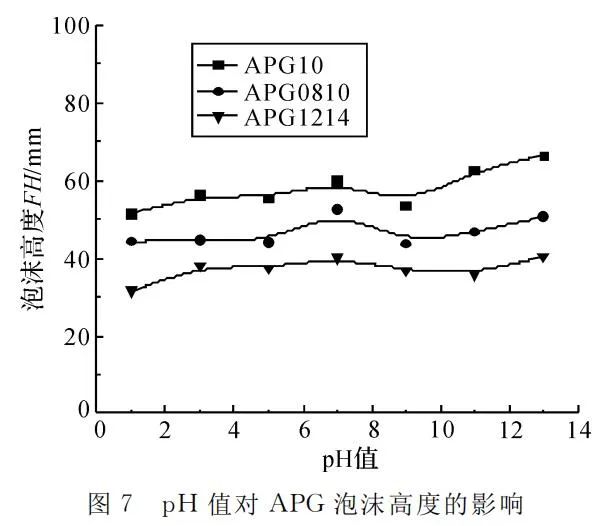
Figure 7 indicates that pH has a relatively small impact on the foaming ability of APG surfactants. The best foaming performance is observed under neutral conditions. Under acidic conditions, as acidity increases, foaming ability slightly decreases, while under alkaline conditions, foaming ability increases.
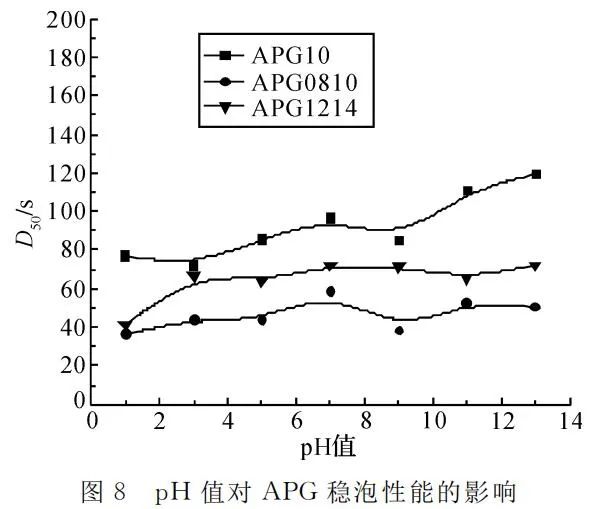
Figure 8 reveals that under acidic conditions, with increasing acidity, stable foaming decreases, while under alkaline conditions, with increasing alkalinity, stable foaming improves. Neutral conditions provide relatively good stable foaming, and under strong alkaline conditions, stable foaming is close to neutral. Acidic and alkaline conditions may disrupt the hydrogen bonds formed between APG's hydrophilic glucose moiety and water molecules, reducing the apparent viscosity of the foam and affecting foam stability. However, under strong alkaline conditions, when the concentration of sodium hydroxide is sufficiently high, it increases the viscosity of the foaming solution, slows down the foam drainage rate, and is favorable for foam stability. With a higher concentration of Na+, foam stability also increases.
Impact of Hard Water on APG Foam Performance
Setting the temperature at 25℃ and APG mass concentration at 4g/L, foaming was conducted using hard water with a calcium carbonate hardness of 0.342g/L and deionized water. Foam height and the time for 50% foam drainage were measured, as shown in Figure 9 and Figure 10.
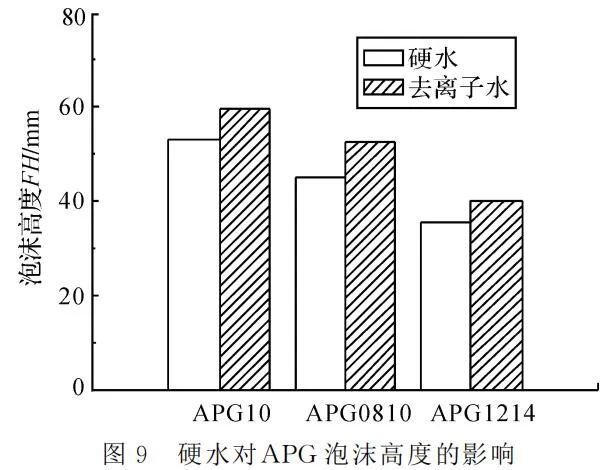
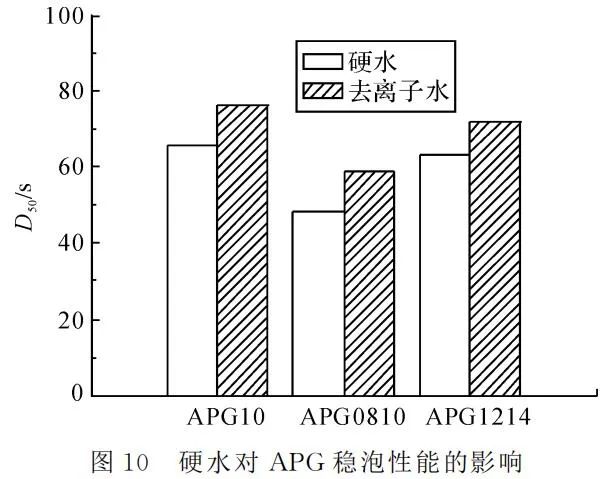
Figures 9 and 10 demonstrate that APG foam performance is influenced by hard water, leading to a decrease in both foaming and stable foaming. This is because APG surfactants exhibit a certain negative charge in the solution, and the presence of divalent metal ions, such as calcium and magnesium, significantly hinders some APG micelles, causing them to aggregate and form larger micelle clusters. This ultimately reduces the foam performance of APG.
Monsa focuses on the production and supply of high-quality surfactants, lipid chemicals, and cosmetic ingredients. Our APG surfactants, derived from renewable resources, are efficient and gentle, injecting environmental friendliness into your cleaning products. Feel free to inquire for more information as we collaborate towards a greener future.
| Product Name | INCI Name | CAS No. | Appearcance | Content |
| MONSA® APG 0810-50 | Alkyl Polyglycoside
| 68515-73-1
110612-47-9 | Light yellow liquid | ≥50 |
| MONSA® APG 0810-60 | ≥60 |
| MONSA® APG 0810-70 | ≥70 |
| MONSA® APG 0814-50 | ≥50 |
| MONSA® APG 1214-50 | ≥50
|






















