Alkyl polyglucoside, abbreviated as APG, is synthesized from naturally derived renewable resources such as natural fatty alcohols and glucose. It is a new type of non-ionic surfactant with comprehensive performance. Combining the characteristics of common non-ionic and anionic surfactants, it has high surface activity, good ecological safety, and solubility, making it the internationally recognized preferred"green" functional surfactant.
Its advantages include rich and delicate foam, good foam stability, strong compatibility, significant synergistic effects, broad-spectrum antibacterial activity, strong alkali resistance, and salt resistance, among others. Therefore, the application fields of alkyl polyglucosides are very wide, such as detergents, cosmetics, biochemistry, food additives, pesticide synergists, etc.
The structure of alkyl polyglucosides
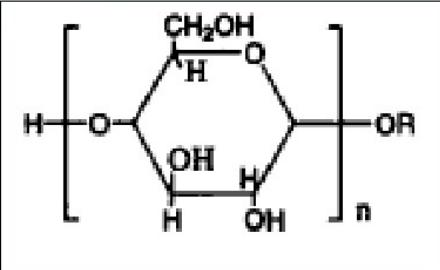
Where:R is an alkyl chain of C8-C10, and n is the average degree of polymerization.
WhenR<c8, the performance of alkyl polyglucoside is poor, and when < span=""></c8, the performance of alkyl polyglucosides is poor, and when <>, while R= C8-C16, its performance is excellent.
Properties of alkyl polyglucosides
Physical characteristics and solubility APG products are mostly made as 50% to 70% aqueous solutions, pure APG is a white powder, but the actual product is cream-colored, light yellow to amber-colored. Its physical properties are closely related to the alkyl carbon chain, the type of sugar used in synthesis, and the degree of polymerization, among other factors. The melting point of APG increases as the carbon chain in the product molecules lengthens, and in some cases when it has not melted, it starts to decompose, indicating that alkyl polyglucosides are prone to decomposition and discoloration when heated. APG is a hygroscopic solid, generally soluble in water, and readily soluble in common organic solvents, exhibiting excellent compatibility, stability, and surface activity in acidic and alkaline solutions, especially in active solvents with a high inorganic content.
Biodegradability
The ability to be biodegraded by microorganisms is an important elimination mechanism to prevent surfactants from accumulating in the environment to dangerous concentrations.alkyl polyglucosides are synthesized from starch and its hydrolysis products with fatty alcohols. In nature, alkyl polyglucosides can be completely biodegraded, without forming metabolites that are difficult to degrade, thereby avoiding creating new pollution in the environment.
Cleansing Ability
The cleansing ability of surfactants varies with changes in ion type, washing conditions, and type of dirt. Sebum dirt on polyester/cotton fabrics is sensitive to non-ionic surfactants. APG shows equivalent cleansing effects to fatty alcohol polyoxyethylene ether sodium sulfate (AES) and is superior to linear alkyl benzene sulfonate sodium (LAS), AS, and SAS.
Surface Activity
The fatty alcohol alkyl chain provides a non-polar hydrophobic group, hence, alkyl polyglucosides usually require an alkyl carbon chain length greater than 8 carbons to exhibit surface activity and critical micelle concentration (CMC). As the alkyl carbon chain lengthens, the surface tension significantly decreases, and the CMC value also decreases, indicating a significant increase in activity. According to literature, when compared experimentally with fatty alcohol polyoxyethylene ether (AEO) and LAS, twelve fatty alcohol alkyl polyglucosides have excellent surface activity.
Synthesis Method of alkyl polyglucosides
Alkyl polyglucosides are mainly prepared through the acid-catalyzed Fischer glycosylation reaction.
Fischer method. This reaction is carried out in alcohol as the solvent, with the sugar prepared in a solution or suspension in alcohol under acidic catalysis, resulting in a mixture of multiple isomers, including different cyclic structures, end-group isomers, and a small amount of linear sugars. When using glucose as the starting material, a short reaction time primarily produces furanosides, and a longer reaction time mainly leads to pyranosides. Prolonged reaction times can also transform the products into more thermodynamically stable α-end-group isomers.
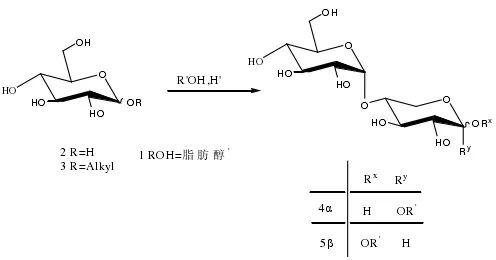
First, monosaccharides (such as glucose, without protecting groups) react under acidic conditions with long-chain fatty alcohols (C5-C18) to produce alkyl monoglycosides, which then polymerize in situ with the remaining monosaccharides to generate alkyl polyglycosides APGs. Since the dehydration reaction generates water, the hydrolysis of the glycosidic bond in APGs is in dynamic equilibrium with its polymerization reaction, and APGs can have an average of 1.5-2.1 sugar units per alkyl chain, making it a challenge to increase the polymerization of sugar units on the alkyl chain of APGs (DPn). Furthermore, after the reaction, distillation is usually required to remove excess fatty alcohols, and an increase in temperature during distillation can also lead to the hydrolysis of some glycosidic bonds in APGs. Enzymatic catalysis generally allows the synthesis of larger DPn APGs, but the expensive cost of enzymes and slow reaction limit their large-scale production.